Science That Changes Lives
From breakthroughs in the lab to real-world progress—accelerating research that delivers results for families today.
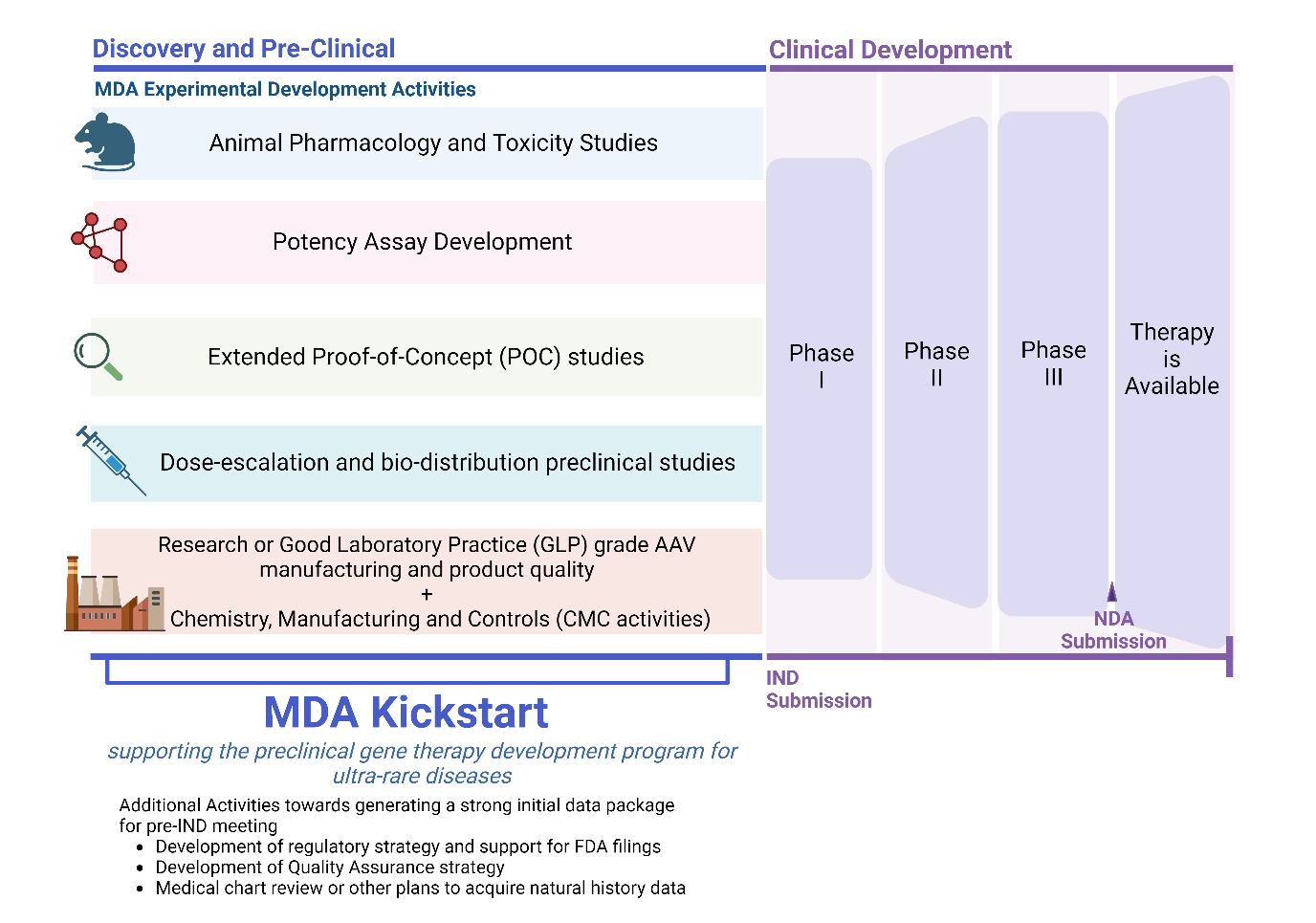
MDA Kickstart Program
MDA advocates for over 300 neuromuscular conditions, with 95% of these affecting less than 1000 individuals in the United States. For therapies to effectively reach the patient community, they traditionally require transitioning from the academic research space to a commercial drug development pipeline; but, because of the small market sizes of many of these diseases, they have only marginal, if any, commercial value. The MDA's Kickstart Program aims to overcome the lack of commercial incentive by bridging the translational gap between early academic science and commercial drug development and leveraging regulatory incentives like the Rare Pediatric Voucher program, to help to de-risk gene therapy programs enough to bring them over the threshold for profitability. MDA’s Kickstart Program brings together a collaborative team consisting of in-house and external experts with research, clinical and regulatory expertise to guide gene therapy programs through the drug development process. The program will be scaled across multiple diseases through sharing of resources including master service agreements and regulatory learnings. Kickstart will document lessons learned to provide a blueprint for other advocacy organizations who share an interest in pursuing drug development for very rare diseases.
The MDA Kickstart Team
-

Sharon Hesterlee, PhD
MDA Chief Research Officer
Accomplishments
- GTx project lead for giant axonal neuropathy, Canavan disease and Friedreich's ataxia in Pfizer's rare disease research unit
- CEO of Lion Therapeutics, a special purpose entity focused on GTx for muscle disease
- EVP Portfolio development for Askbio - supported in-licensing and BD efforts; helped raise $235M series A
-

Angela Lek, PhD
MDA Vice President, Research
Accomplishments
- Completed postdoctoral studies at Harvard Medical School, faculty member of Yale School of Medicine
- Consultant for GTx programs with NIH and rare disease advocacy organizations
- Expertise in cell and animal models of neuromuscular disease
- Provides strategic direction for MDA research activities and venture philanthropy program
-

Marina Kolocha, PharmD, PMP
Independent Contractor Project Manager
Accomplishments
- 18 years of drug development expertise and roles in the development of over 242 rare and orphan assets
- Executive Director Syneos One Rare and Orphan, Corporate, Syneos Health
- Sr Director Therapeutic Strategy and Client Solutions, INC Research
-

Scott Kozak
MDA Vice President Research Business Development
Accomplishments
- 30 years of experience in business development in the health sciences industry
- Yale Ventures - Entrepreneur in Residence
- Co-Founder and CEO of several biotech companies
- In-licensing, out-licensing, raising investment funds
Kickstart is also assisted by an advisory committee consisting of gene therapy research, clinical and industry experts.
Kickstart pilot case: congenital myasthenic syndrome due to ChAT deficiency
The pilot project for MDA’s Kickstart program focuses on the development of a gene therapy for a form of Congenital Myasthenic Syndrome (CMS) caused by mutations in the Choline acetyltransferase (ChAT) gene. Disease-causing mutations in the ChAT gene cause disruptions in signal transmission from nerve cells to muscles, and result in muscle weakness and potentially fatal apnea. CMS due to ChAT deficiency (CMS-ChAT) affects about 100-150 children and adults in the United States and is characterized by muscle weakness and life-threatening episodes in which breathing stops. This program is a collaboration with Dr. Ricardo Maselli (UC Davis) who is a world recognized clinician and researcher in congenital myasthenic syndromes. Recently, Dr. Maselli published results on restoration of ChAT-deficiency in mice using adeno-associated virus (AAV) gene therapy (pubmed.ncbi.nlm.nih.gov/38299967). The gene therapy technology established by Dr. Maselli forms the basis for our Kickstart drug development program. MDA is currently working with Dr. Maselli to conduct the necessary studies to meet an early FDA milestone (pre-IND) for drug development.
About CHAT-CMS
Congenital Myasthenic Syndromes (CMS) are a diverse group of neuromuscular disorders affecting the neuromuscular junction, the site where nerve signals are transmitted to muscles. Approximately 5% of CMS cases are associated with mutations in the choline O-acetyltransferase (CHAT) gene, which encodes an enzyme crucial for synthesizing acetylcholine, a neurotransmitter essential for muscle contraction (See Figure below). Mutations in CHAT lead to reduced acetylcholine production, impairing communication between nerves and muscles. This disruption can cause symptoms ranging from mild muscle fatigue and occasional sudden loss of muscle tone to severe muscle weakness, potentially leading to respiratory failure and bulbar deficits that require tracheostomy, continuous mechanical ventilation, and lifelong gastric tube feeding. The severity of CHAT-CMS varies greatly among individuals, with symptoms typically appearing in infancy or early childhood. A key feature of CMS is the rapid onset of muscle fatigue, which can occur within minutes, unlike the slower fluctuations seen in other neuromuscular disorders. This rapid fatigue can lead to life-threatening apneas. Management focuses on symptomatic relief, often using medications that enhance neuromuscular transmission, such as acetylcholinesterase inhibitors, along with supportive therapies and regular monitoring to improve quality of life and symptom control.
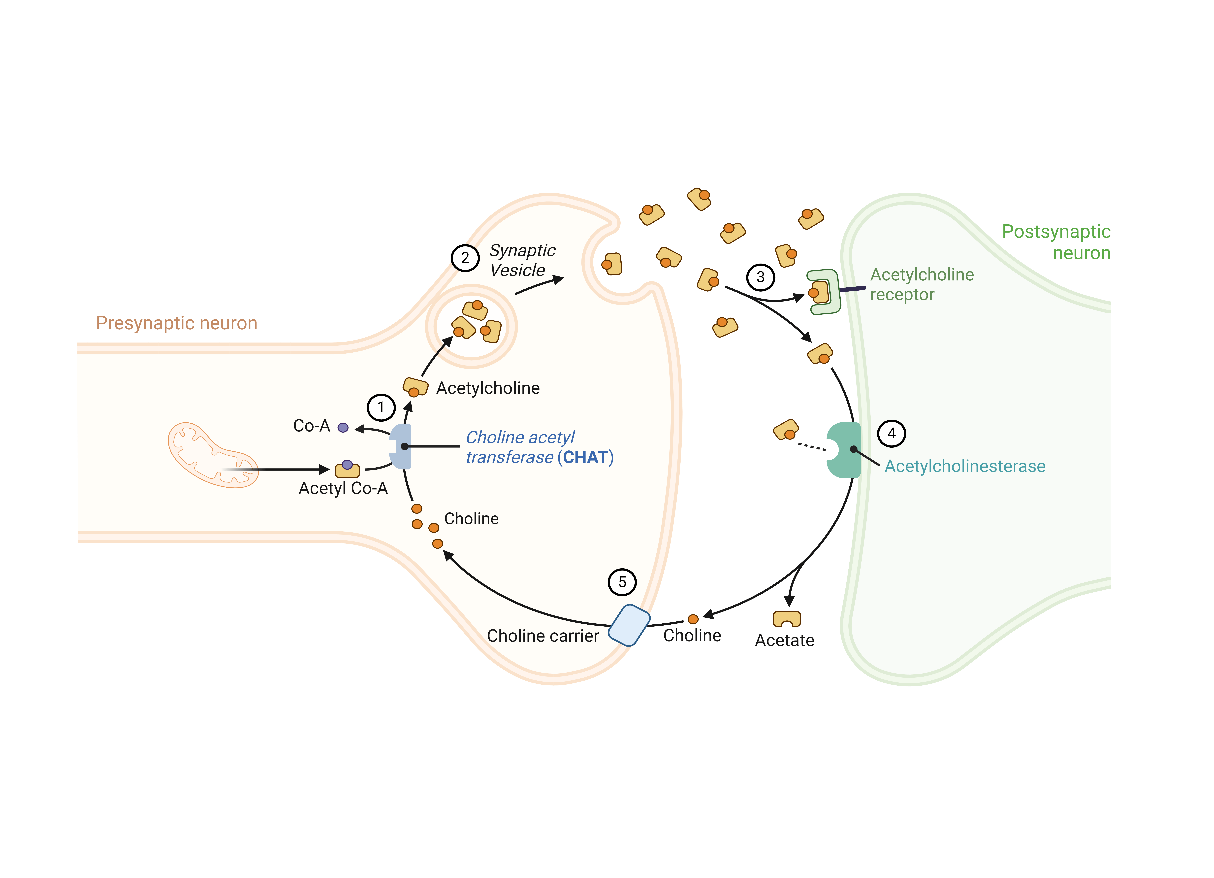
The research objectives of the ChAT program are the following:
- Design CHAT constructs with three different candidate promoters and perform validation of CHAT expression
- Conduct umbrella study in vivo to determine top candidate construct for advancement into the clinic
- Conduct dose-finding study with selected candidate construct to determine optimal dose for IND-enabling studies
- Evaluate a preliminary safety profile of the selected construct to guide design of IND-enabling tox studies
- Develop and validate an in vitro potency assay based on enzymatic activity of CHAT
- Conduct an informative pre-IND meeting with the FDA
- Evaluate availability of natural history data and conduct benefit/risk assessments with this population
For more information about MDA’s Kickstart Program, please reach out to grants@mdausa.org.
- MDA Medical Education
- Grants at a Glance
- Research Grants
- Creating a New Therapy
- What We've Achieved
- MDA Venture Philanthropy
- MOVR
- MDA Annual Conference
- Newborn Screening for Neuromuscular Diseases
- Cost of Illness of Neuromuscular Diseases in the US
- Contact Our Research Team
- MDA Kickstart Program
- Telemedicine Resources
Find MDA
in your Community
-

Search for Clinical Trials
Learn More -

Grants at a Glance
Read More