
The Brain in MMD

Specialists, support groups and stimulant medications help families cope with the unusual cognitive and personality effects of type 1 myotonic dystrophy
*Many people interviewed for this article asked not to be identified to protect the privacy of affected family members.
“Everybody knows the word apathy,” says a California woman whose 25-year-old daughter’s type 1 myotonic dystrophy (MMD1, sometimes called DM1) was diagnosed just a few years ago. “People use the word loosely. I don’t think it does justice to the reality of this disease.”
As often happens, the young woman’s MMD1 wasn’t recognized until she gave birth to a child with the severe congenital-onset form of the disease. Says her mother, “Apathy itself, the absence of emotion, doesn’t really capture all the nuances. She’s 25 but functioning like a child or a teenager. If you can’t think beyond the moment, then how can you be motivated?”
In a Dallas suburb, S.M., an administrator at an insurance company, has coped for more than two decades with caring for her husband and two children with MMD1. “She’s doing nothing right now,” she says of her 20-year-old daughter, whose diagnosis came in her freshman year of high school. “She doesn’t really have any skills, and I can’t get her motivated to get skills.”
She and her husband didn’t learn about his MMD until he was in his 30s. “We just thought he was moody or lazy or afraid of being around people,” S.M. says. “I took him to doctor after doctor, psychiatrist after psychiatrist.” Finally, after a fall, a neurologist examined him and made the diagnosis.
Her son, whose disease is the congenital-onset form, was “really slow at everything” from birth, although his disease wasn’t diagnosed until he was 8. “He wasn’t potty-trained until he was almost 4. He started kindergarten at 5, and that was horrible. He couldn’t sit still. He would fall a lot.”
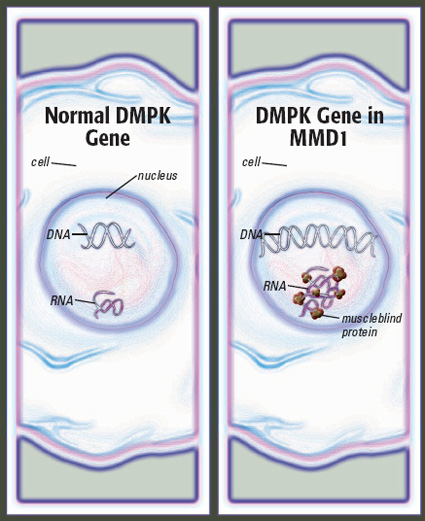
Blame it on expanded DNA
Ever since the first descriptions of myotonic dystrophy early in the 20th century, references have been made not only to its characteristic weakness, muscle atrophy (shrinkage) and myotonia (difficulty relaxing muscles), but to particular personality characteristics and sometimes reduced intelligence that can occur.
 In the 1990s, the disease now known as type 1 MMD was found to result from an expanded stretch of DNA on chromosome 19, in a gene known as DMPK. The expansion, through complex mechanisms that aren’t entirely clear, affects voluntary muscles, the muscles of the gastrointestinal tract, the heart, the eyes and the brain.
In the 1990s, the disease now known as type 1 MMD was found to result from an expanded stretch of DNA on chromosome 19, in a gene known as DMPK. The expansion, through complex mechanisms that aren’t entirely clear, affects voluntary muscles, the muscles of the gastrointestinal tract, the heart, the eyes and the brain.
The expanded DNA consists of repeated sequences of cytosine (C), thymine (T) and guanine (G), and its length is measured in numbers of “CTG repeats.” In general, more CTG repeats mean more severe disease and earlier disease onset.
The average person has between five and 37 CTG repeats. Numbers in the hundreds generally cause classic MMD1, with moderately severe symptoms beginning in adolescence or young adulthood. Repeat numbers of about 1,000 or more generally result in congenital MMD1, a severe disease that’s evident at birth (though often not diagnosed until later).
In 2001, a form of MMD now called type 2 was found to result from a similar DNA repeat expansion on chromosome 3. Much less is known about this form of the disease, and the cognitive and personality effects, though scarcely studied, so far don’t seem severe.
An MMD1 profile?
“What seems to be consistently found in type 1 myotonic dystrophy is impaired visual-spatial memory, a verbal abstract reasoning impairment, and more attention disorders than one might expect by chance,” says Patricia Evans, a child neurologist at Children’s Medical Center, Dallas.
Evans conducts neuropsychological testing on children and adolescents with MMD1 who are referred to her through the MDA clinic. “In addition, you see a significantly higher incidence of mood issues, specifically anxiety and depression.”
Neuropsychological testing, Evans explains, “looks at how people think and feel. Typically an assessment includes a way to measure intellectual strengths and weaknesses, learning disabilities and problems, any attentional disorders and emotional issues.”
Children with MMD1 often have problems “trying to recall or reason through visual-spatial information,” she says, referring to making sense of what you see and having an accurate perception of one’s own position in space and that of people and objects around you. “You have to have visual-spatial reasoning and memory for nonverbal communication and to read faces. And you have to figure out where your body is in space in relation to somebody else’s so you don’t ram into them,” Evans says.
Although parents often are told their MMD1-affected children have an attention deficit disorder, Evans says they really have deficits in “executive function.”
“In the common parlance of schools, it’s called attention deficit disorder, and attention to a task is tougher for these kids,” she says. But executive function is “really a spectrum that involves drive, motivation, focusing, planning and the ability to suppress short-term gains for long-term goals.”
Evans considers all these difficulties as making up a “type 1 myotonic dystrophy profile,” although this profile doesn’t describe everyone and varies in severity.
MMD1 also causes excessive daytime sleepiness, probably because of abnormalities in the arousal center of the brain, which can be misinterpreted as disinterest, depression or laziness.
True mental retardation occurs at the severe end of the MMD1 spectrum, mainly in the congenital form of the disease.
In addition to its effects on the brain, MMD1 can cause weakness of the mouth and tongue muscles, making speech hard to understand, and of the facial muscles, limiting facial expression.
“Step one is understanding that lack of facial expression is not dullness or unresponsiveness,” says Vikki Stefans, a physician specializing in physical medicine and rehabilitation at Arkansas Children’s Hospital in Little Rock, where she sees patients in the MDA clinic.
Like Evans, Stefans identifies executive function deficits as a common feature of MMD1 and tries to get neuropsychological testing for schoolage children with the disease to get a “detailed profile of their strengths and weaknesses in thinking and learning.”
Mood, medications and motivation
Stefans says most young children with MMD1 benefit from physical therapy, occupational therapy, and speech and language therapy.
Later, special education often is helpful. Even children with mental retardation, she says, can still learn. “They may not be able to go as far or as fast, and they may have to have things simplified, repeated and broken down, but that doesn’t mean they can’t learn,” she notes. “You’ve got to undo that translation in everybody’s head.”
The problems of older children and teenagers with classic MMD1 can be more subtle and harder to address. “Just because you’re walking and talking doesn’t mean everything works 100 percent,” she says.
Dealing with executive function deficits, Stefans says, first requires “realizing that you have them and need some help.”
Stimulant medications are a tool she doesn’t hesitate to use for children or young adults who are excessively sleepy during the day. “I’ve had folks do well with Ritalin [methylphenidate] or Adderall [amphetamine],” she says. She also has prescribed Provigil (modafinil), but some insurers won’t cover it until other drugs have been tried and a formal sleep study conducted, she notes.
Stefans cautions that kids with MMD “aren’t immune to emotional ups and downs by any means.” If the problem is emotional, she says, “all the stimulants in the world won’t help.”
 Patricia Evans says she sees adolescents with MMD1 in whom parents can’t “light a fire.” They appear to have no motivation, no apparent drive, and spend hours playing video games.
Patricia Evans says she sees adolescents with MMD1 in whom parents can’t “light a fire.” They appear to have no motivation, no apparent drive, and spend hours playing video games.
This may be due to a combination of lack of organization, difficulty focusing and mood issues, says Evans. “I don’t worry about focusing [problems] until a child’s moods have become more stable. Then you can fix the focusing better.”
Like Stefans, Evans is enthusiastic about medications as an addition to other therapies. She often prescribes a mood-stabilizing drug such as Abilify (aripiprazole) or Risperdal (risperidone), or an antidepressant to alleviate depression prior to addressing cognitive problems.
Counseling is also part of her recommendations. “If I’m using something that changes mood, I like these kids to be in counseling,” she says. “Therapists are going to have a much better feeling of where they are emotionally. Therapists can also be instrumental in coaching a child to have better insight into his or her cognitive and emotional challenges. That’s critically important for long-term success.”
 Tackling focusing without looking at mood sometimes goes nowhere, Evans says. She notes that Ritalin (which she prescribes to help with attention) “will make you more focused, but it won’t make you open a book. Once you open the book, it will help you focus better, but it won’t make you open it in the first place.”
Tackling focusing without looking at mood sometimes goes nowhere, Evans says. She notes that Ritalin (which she prescribes to help with attention) “will make you more focused, but it won’t make you open a book. Once you open the book, it will help you focus better, but it won’t make you open it in the first place.”
Helping parents and teachers reward effort and implement reasonable consequences for inappropriate behavior are helpful strategies. “It’s labor-intensive,” she notes.
Surmounting stumbling blocks
“We battled attention deficit disorder all along,” S.M. says of her daughter, T. “She had a few learning problems but nothing really serious, and there was a lot of immaturity.”
T. was treated with attention-focusing medications like Ritalin, which didn’t solve the problem but may have helped.
Until high school, she was in regular classes, but with modified work, teachers who made sure she understood the lessons, and the option of substituting oral for written assignments.
But the demands of high school proved harder to meet. “We tried to pull her out of regular classrooms,” her mother says, “But she didn’t like that. She felt like she was in the dumb classes. By the end of her freshman year, I started home-schooling her.
“The hardest thing is getting her to focus. Being specific makes a big difference. If I tell her exactly what needs to be done, she gets it done.”
Starting anything was a big stumbling block as well. If T. had a writing assignment, S.M. would help her choose a topic and write a sentence, after which T. could continue on her own. Without such help, her mother says, she would never get started. (She has now graduated from high school.)
Special education in congenital MMD1
For children with congenital MMD1, the problems are different. “Originally he spent half his time in an integrated classroom and half his time in a special resources classroom,” says Lisa Earls of Haskell, Ark., whose 8-year-old son, Chase, has this form of the disease. “But he spends more time each year with the special resources class and less with the integrated class, because the integrated class progresses much more quickly than he does. He needs extra time and attention.”
Chase was slow to develop physically and intellectually, and facial weakness interfered with social development.
 “I think he was probably 9 to 12 months old before I actually could tell he was smiling,” she says. She watches his eyes and how he’s observing other people to detect how he’s feeling.
“I think he was probably 9 to 12 months old before I actually could tell he was smiling,” she says. She watches his eyes and how he’s observing other people to detect how he’s feeling.
At 3, Chase’s IQ was estimated at 74. (An average score is 100.) He’s currently functioning at about a 6-year-old level, Lisa says. “He can read some words. He’s writing now, which is a major improvement. But he’s doing what kindergarteners and beginning first-graders are doing, and he’s in second grade.”
Lisa says she knows his intelligence is moderately impaired, but she also sees fatigue and apparent lack of motivation as factors in his performance and behavior. “If he gets tired or bored, he just stops trying,” she says. “He struggles when he gets to a word he doesn’t know, and he just wants to put the book down and do something else.”
Strattera (atomoxetine), normally prescribed for attention deficit disorder, has helped with excessive sleepiness during the day and waking during the night and has improved Chase’s concentration. “His grades and behavior showed a dramatic improvement when he started taking it,” Earls says.
She and his teachers rely heavily on rewards as motivational tools. “The computer is a huge motivation for him,” she says. “Sometimes he doesn’t want to do any of his work. The teacher and I have discussed different ideas. He had a spelling list that he wrote very neatly with wonderful handwriting. The teacher made a huge deal of it, and we hung it up on the refrigerator. Now he wants to bring home another paper to get it up on the refrigerator.”
T.’s mother, S.M., has a 17-year-old son, D., with congenital MMD1. “He has many learning issues and may never be more mature than a 12- or 13- year-old,” she says.
After years of struggling with D.’s respiratory problems, severe constipation, learning disabilities and attention deficits, S.M. got help from neurologist Susan Iannaccone at the MDA clinic at Texas Scottish Rite Hospital for Children in Dallas when D. was about 8. (Iannaccone is now the MDA clinic director at Children’s Medical Center in Dallas.) “She saved my life many times,” S.M. says.
D. went to the regular public elementary school for a while but had trouble keeping up. “We pulled him out and put him in a charter school, where we explained everything to them, and they worked with D. from day one. There were about eight students in his class, and the teacher could pull him aside easily. He did fine as long as they worked with him. They didn’t give him as much work as the other kids got.”
When obstacles began surfacing early in high school, S.M. began home-schooling D. She also talked with a psychologist, who advised her that D. might best be served by concentrating on practical life skills rather than on academics.
The school sends a tutor once a week. At home, 17-year-old D. is learning to cook, balance a checkbook, go to the grocery store, do yard work and pool maintenance, and even drive a car. “He’s really a good driver, very conscientious,” S.M. says, although she worries about his driving.
“As long as you’re patient, you don’t have to be as specific with him as with T.,” she says, “but you have to be specific. You can’t just say, ‘Clean up the backyard.’ You have to say, ‘Sweep the porch.’ He tries. He thinks about it.”
The solutions for D. were different than for T., says his mom. “He’s never going to have an actual high school education. He’s not going to go through a lot of the classes most people go through.”
A different life
At a recent conference sponsored by her support group (see Not the Only Ones below), Alice Gunderson recalls, a young man addressed the parents. “He said, ‘Please don’t give up on us. It may take longer for us to get things done, but we want to be part of the living.’”
Evans also believes there’s often more beneath the surface of young people with MMD than meets the eye, and sometimes she can get to it by talking with the young person without his or her parents.
Stefans says she tends to form long-term bonds with her MMD1 patients. “They’re some of my favorite kids,” she says. “I guess anybody who’s living a different life and finds a way to do it well is impressive.”
Solutions from Science?
Although behavioral and cognitive abnormalities have been observed in MMD1 since the disease was first described, disease-related changes in the brain that might account for them have been a complete mystery until recently.
In 2000, a French research group led by Genevieve Gourdon at the Necker Hospital for Sick Children in Paris, bred mice with expanded CTG repeats in the DMPK gene, precisely the same genetic defect that causes MMD1 in humans. The following year, when they examined the brains of mice carrying at least 300 repeats in the DMPK gene, they saw abnormally formed molecules of the protein tau (rhymes with “now”), a protein long suspected of causing trouble in the brain in Alzheimer’s disease and other degenerative conditions.
Then, in April, members of the French team and others, including MDA-supported Thomas Cooper at Baylor College of Medicine in Houston, reported their observations of human MMD1-affected brains. In these, they found clumps of shortened tau molecules in cases where there were very long DNA expansions.
Cooper and his colleagues are also breeding mice that have an expanded stretch of DNA in the same gene as humans with the disease. They’re planning to control the gene’s activity in different tissues, including the brain, to measure its effects.
Cooper thinks abnormal tau is likely important but that it may not explain everything. For one thing, he says, MMD-affected patients don’t act like patients with Alzheimer’s disease or other tau-related disorders. In MMD, tau may only be abnormally formed in some brain cells and not others, he speculates, while in Alzheimer’s the abnormalities are widespread.
Maurice Swanson, a molecular biologist and MDA grantee at the University of Florida in Gainesville, is also studying MMD’s effects on the brain.
He’s interested in the tau protein, among others, but his group has concentrated on three proteins known as muscleblind 1, 2 and 3.
These proteins stick to and become trapped by long strands of repeated sequences of RNA, a compound made from DNA’s instructions, in each MMD1-affected cell nucleus.
Swanson’s team has bred mice that don’t make muscleblind 1 and found they make a short version of tau more often than they should, just as some humans with MMD1 do.
Mice missing just one of the three muscleblind proteins show many of the symptoms of MMD1, Swanson says, including myotonia and cataracts. He suspects that mice missing all three muscleblind proteins might demonstrate all the features of MMD1.
Swanson says that so far, there haven’t been enough comprehensive analyses of MMD1-affected brains, but those that have been done show loss of cells in the frontal lobe, the part of the brain that controls decision making, problem solving and planning (executive function).
In Swanson’s lab, scientists are examining the brains of muscleblind-deficient mice to see what differences they may find from normal mouse brains.
Asked whether he sees any hope of altering or preventing the brain effects of MMD1, Swanson is optimistic. “Absolutely,” he says. Blocking the long sections of repeated RNA, the root cause of the multisystem problems in MMD, is becoming increasingly feasible with new molecular strategies known as “RNA interference” and “antisense.”
He also thinks raising the levels of muscleblind proteins in MMD1 patients, using either gene therapy or small-molecule drug approaches, might prove beneficial.
Not the Only Ones
More than a decade ago, when Alice Gunderson’s husband, Ed, was 48, they learned he had MMD1. As Ed’s condition worsened, the couple started attending a general support group sponsored by MDA near their home in the Los Angeles area.
Ed had some practical questions, like how to get up off the floor after a fall. The support group, however, included people with severe disabilities and parents of children with life-threatening diseases, whose questions were different from Ed’s. “I don’t have the heart to ask about getting up off the floor when there are people in the room who would love to stand up and have the opportunity to fall,” he told Alice.
That’s what made her start thinking about an MMD-specific support group. She turned to her local MDA health care service coordinator for help, and a meeting was organized, to which two people came. Ten people came to the second meeting. More than 30 showed up for the third.
“People were standing at the door waiting to get in,” recalls Alice. “I said, ‘I think we’ve hit on something.’”
Today, the Gundersons continue to work with MDA’s Los Angeles chapter to offer a support group program for those with myotonic dystrophy.
“Our mission is education, support and fundraising for MDA-supported research in myotonic dystrophy,” she says. “We want people to feel more in control of their disease and not like victims of it.”
Parents can “just get worn down” by the challenges of a child with MMD1, she says. Especially for parents of children with congenital MMD1, talking with each other, particularly about the cognitive aspects of their child’s disease, is helpful.
Once parents get past the health crises of the first two years, Gunderson says, “it gets to be about what they can do to get them into the educational system. ... Families find ‘aha’ moments from one another. They find out they’re not the only ones.”
The social limitations of MMD are a reality that must be faced, she believes. “The majority of young people with the disease have cognitive and personality issues and don’t really feel they fit in,” Gunderson says, “so support groups and other meetings can be a safe haven. Sometimes they can say things there that they can’t say to their parents. They’re not excluded. They’re included.”
MDA Resource Center: We’re Here For You
Our trained specialists are here to provide one-on-one support for every part of your journey. Send a message below or call us at 1-833-ASK-MDA1 (1-833-275-6321). If you live outside the U.S., we may be able to connect you to muscular dystrophy groups in your area, but MDA programs are only available in the U.S.
Request Information